level 5 science

The functional properties of Manuka honey are partly attributed to the proteins present in the honey. The proteins are major royal jelly proteins (MRJPs). Other proteins are also present. The structure of the protein complex has been determined and shown above. The geometry of the structure has a 2 fold symmetry which produces some unique features of the protein complex. MRJP1 plays a number of roles in bee development. The clinically proven benefits of bee proteins in human health and well-being are at the level of human case studies.
However, Manuka honey has gained traction as a wound healing medical device in the treatment of chronic wounds as it has antimicrobial properties as well as anti-inflammatory properties, which contribute to its use in wound healing.

The way Manuka honey works has been attributed to MGO and its reactive properties allowing it to inhibit bacterial growth. However, the story is somewhat more confusing and unexpected in all honesty. The reason for the complexities is that MGO levels only correlate with antibacterial activity as if it is a good surrogate marker for the true antibacterial ingredient. There is also synergy where the antibacterial properties can increase in the presence of phenolics.

The Manuka tree grows as a pioneering plant in locations that have low quality soil. The soil has a high content of iron which translates to the Manuka honey containing an abundant amount of iron.

So the unique composition of iron, phenolics (plant antioxidants) and New Zealand's light spectrum with the ozone whole, with its increased UV levels means we have the conditions that are ideal for Manuka honey to form and perform its unique chemistry. The unique chemistry that occurs in the Manuka pollen is based on a light based storage of energy not unlike photosynthesis where light is captured by pigments in plant cells in organelles called chloroplasts but a photo-reduction of mineral iron that is bound to the phenolic aromatic ring pi electrons. This light based reaction causes iron to go from Fe3+ to Fe2+.
Here we can see the three components of the Manuka honey at play, New Zealand's high incidence of UV light, phenolics and iron. These elements come together to react in a way to produce highly reactive Fe2+. This forms what is known as the photo-reduction step in the energy system present in Manuka honey. This is occurring in pollen grains within the Manuka honey.

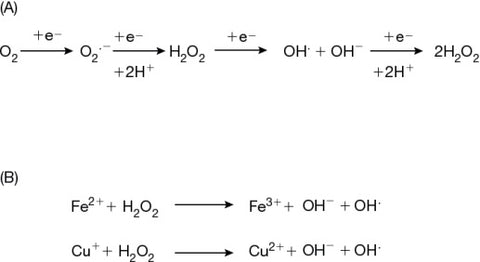
The other side of the Manuka honey story is connected to glucose oxidase activity and its role in the generation of hydrogen peroxide. This enzyme is an important role to play in the antibacterial story of honey. The production of hydrogen peroxide can only occur when honey is diluted with water so the reaction can proceed. Without the addition of water the activity of this enzyme is prevented. As soon as water is added the enzyme starts producing hydrogen peroxide.
The interaction between these two components Fe2+ and hydrogen peroxide produces a short lived high energy electron with a half-life of 1 billionth of a second or a nanosecond. This chemical is called a hydroxyl radical and the reaction system is known as photo-fenton chemistry. The energy within this short period of time is 1200 electron volts and it enables the breakdown of proteins, lipids and carbohydrates into carbon dioxide and water. This reactive chemical is naturally produced in our body during apoptosis, part of our bodies natural regeneration system.
The generation of such high energy short lived electrons in Manuka honey by this system is what is responsible for the antibacterial activity. If you want to dig a little deeper continue to level 6.


